The overall goal of my research is to develop gene-based therapies for diseases of the brain and eye. My approach is to use rAAV (recombinant adeno-associated virus) to deliver therapies to treat mouse models of human disease. Currently, we are mining the human genome for cell-type-specific promoters to use in viruses, and developing gene augmentation and genome editing (CRISPR/cas9) therapies focused on curing the congenital blindness aniridia.
GENE THERAPY FOR ANIRIDIA
Our goal is to develop an rAAV-based gene therapy for aniridia. Our experiments are the first towards rAAV-based gene therapy for aniridia in mouse.
Aniridia is a rare, congenital, panocular, eye disorder. Individuals with aniridia are typically born with low vision, and because there is no effective treatment, subsequently lose all vision. Thus, there is a therapeutic window available to prevent vision loss. Approximately 80% of individuals with aniridia are known to carry a mutation affecting one copy of the transcription factor PAX6 (paired box gene 6). Our long-term goal is to make gene therapy an option for patients with aniridia. Our short-term goal is to cure the mouse model of aniridia. Curing aniridia in mice will lay the conceptual and practical foundation upon which human gene therapy can be designed.
Gene therapy uses nucleic acids to alter cellular behavior and treat disease. After 25 years of research, the promise of gene therapy is being realized; currently there are 24 rAAV-based ocular clinical trials ongoing. The eye is an excellent organ for gene therapy because it is easily accessible. Currently we are taking a two-pronged approach to gene therapy for aniridia: augmentation gene therapy, and genome editing using CRISPR (clustered regularly interspaced short palindromic repeats) /cas9 (CRISPR-associated nuclease 9).
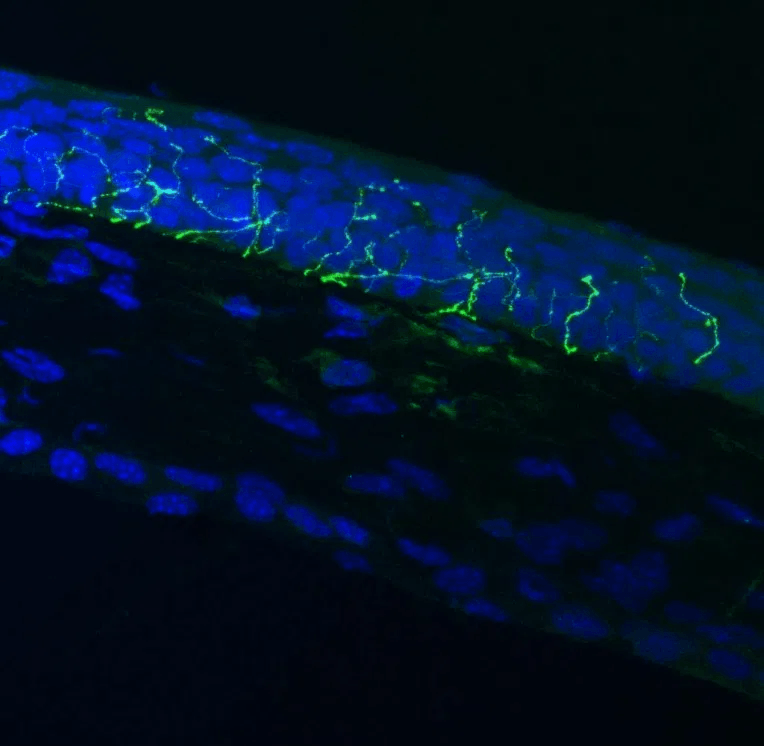
For augmentation gene therapy, we are delivering additional PAX6 encoded in rAAV directly to the mouse eye. Because PAX6 may need to be carefully regulated, we have designed “MiniPromoters” (small selected regions of human promoters) from the human PAX6 gene and tested them in the mouse eye (Hickmott et al., 2016 in revision). Two PAX6-MiniPromoters drove expression in three of the four cell types that express PAX6 in the adult mouse retina. Combined, they capture all four-cell types, making them potential tools for research, and PAX6-gene therapy for aniridia. Successfully using a transcription factor to treat aniridia will not only provide a treatment for this disorder, but further clear the path for other rare disorders, and for the first time open up many transcription factor diseases for gene therapy.
For genome-editing therapy, we are using the bacterial CRISPR/cas9 system. It will be delivered in rAAV to edit genomic DNA in vivo in a mouse model of aniridia. Thus, we will merge the safe and efficient access to a variety of cell types achieved by AAV, with the precision of CRISPR/cas9 editing. This base-pair substitution strategy would be applicable to 62–86% of classical aniridia patients. The final therapy would be “precision medicine”, in that a different CRISPR/cas9 gene-editing assay would be needed for each patient.

HUMAN MINIPROMOTERS FOR RESTRICTED EXPRESSION OF OCULAR AND BRAIN GENE THERAPY
We still have only a cursory understanding of the 98% of the human genome that is non-coding. However, we do know that it contains large complex promoters, often with regulatory modules dispersed throughout the gene. Understanding and harnessing this regulatory potential into “MiniPromoters” (small selected regions of human promoters), to drive gene expression in defined regions of the brain and eye, is an interest of Dr. Simpson and her colleagues. Such MiniPromoters have many applications for basic and clinical research, but the most demanding and exciting is gene therapy; a promising approach for diseases with unmet therapeutic needs. By being cell type specific, MiniPromoters are designed to increase gene therapy safety by reducing off-target side effects, while increasing efficacy by broadening the pallet of therapeutic proteins that can be safely delivered.
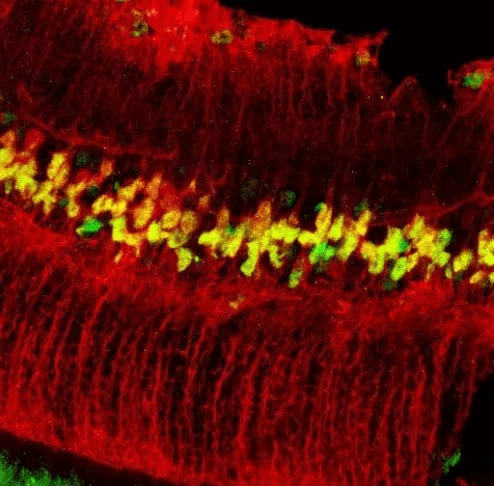
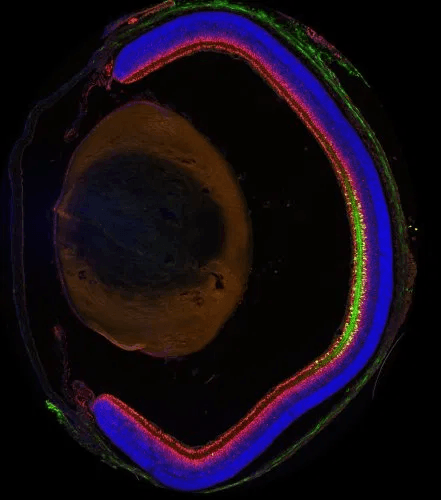
We have taken steps towards filling the need for MiniPromoters by developing a high-throughput pipeline that goes from genome-based bioinformatic design to rapid testing in vivo. For much of this work, therapeutically interesting Pleiades MiniPromoters, which were ~4 kb and previously tested in knock-in mice (de Leeuw et al., 2014), are being “cut down” to ~2.5 kb and tested in recombinant adeno-associated virus (rAAV); the virus of choice for gene therapy of the central nervous system. The data has shown that 16 of the 19 (84%) MiniPromoters recapitulated the expression pattern of their design source. This included expression of: Ple67 in brain raphe nuclei; Ple155 in Purkinje cells of the cerebellum, and retinal bipolar ON cells; Ple261 in endothelial cells of brain blood vessels; and Ple264 in retinal Müller glia. Overall, the methodology and the resulting MiniPromoters are important advances for basic and preclinical research, and may enable a paradigm shift in gene therapy.
Currently these genome studies to design MiniPromoters are being funded by Brain Canada/CQDM for a project entitled: “Human MiniPromoters for Restricted Expression of Ocular Gene Therapy”. The main deliverable is a toolkit of human MiniPromoters with restricted expression suitable for ocular gene therapy. The toolkit will include 18 MiniPromoters, fully characterized in mouse for overall CNS, and detailed eye, expression. In total, 48 viruses carrying 30 MiniPromoters will be studied; with an expected success rate of 60%. The three most therapeutically promising will also be fully characterized in the monkey eye. Their most avant garde MiniPromoter recently enabled a previously unsuccessful rAAV gene therapy to restore visual function in a mouse model of congenital night blindness (Scalabrino et al., 2015). Thus, these MiniPromoter tools will be positioned for investment by biopharmaceutical companies interested in delivering therapeutic molecules to the eye.
These tools are being distributed worldwide via The Jackson Laboratory (75 mouse strains) and Addgene (366 plasmids, 260 requests).